Abstract
Targeting B-cell maturation antigen (BCMA) with antibody drug conjugates (ADCs), bispecific antibodies, or chimeric antigen receptor (CAR) T-cells is an established strategy in relapsed/refractory multiple myeloma (RRMM). In most European countries, the respective agents are approved after exposure to at least three lines of therapy including a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and an anti-CD38 antibody. It still needs to be clarified whether sequential treatment with different anti-BCMA directed agents is safe and effective. In some countries, payers do not cover/reimburse CAR T-cell treatment directed against BCMA if the patient was previously exposed to an anti-BCMA ADC or bispecific antibody. To the best of our knowledge, we describe the first case of rapid response to BCMA-directed CAR-T cells (idecabtagene vicleucel; Ide-cel) in a heavily pretreated patient who had already failed two prior lines of anti-BCMA directed therapies.
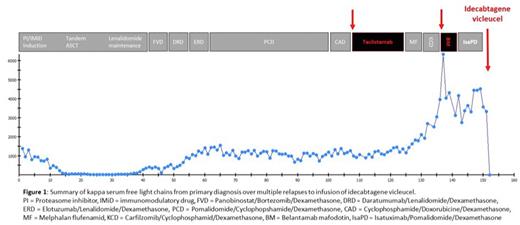
A 54-year-old female with IgG kappa MM harboring chromosomal deletion 17p and gain of 1q21 presented to our department. Initial treatment consisted of PI/IMiD-based induction therapy, high-dose melphalan as part of tandem autologous stem cell transplantations followed by lenalidomide maintenance. Very good partial remission (VGPR) lasted 3 years, and the patient became triple-class (PI, IMiD, anti-CD38)-refractory only two years after first relapse. We enrolled the patient in a phase I/II trial of the anti-BCMA bispecific antibody teclistamab. Cytokine release syndrome (CRS) grade III complicated teclistamab treatment and the patient showed biochemical relapse and progressive osteolytic lesions after being 4 months on study. After failing two subsequent alkylator-based lines of therapy including melphalan flufenamid, the patient was treated with belantamab mafodotin and progressed after two months. Treatment was switched to isatuximab/pomalidomide/dexamethasone. The latter combination represented the 11th line of therapy for this patient. After approval of Ide-cel in Germany in 2021, leukapheresis for successive CAR T-cell production was performed. Since the patient showed a partial response after 1 month, this bridging therapy with isatuximab/pomalidomide/dexamethasone was continued until successful CAR manufacturing. Unfortunately, the patient experienced disease progression prior to lymphodepletion therapy with progressive transfusion-dependent pancytopenia and subtotal infiltration of bone marrow by malignant plasma cells. PET/CT showed no evidence of extramedullary disease. Lymphodepletion with fludarabine/cyclophosphamide according to the phase-II KarMMa protocol was initiated, and 441.5 x 10exp6 CAR T-cells were infused. The inpatient stay was complicated by grade I CRS and septicemia due to gram-negative bacteria requiring vasopressor treatment for 48 hours. Two weeks after infusion of Ide-cel, significant increases of serum uric acid and lactate dehydrogenase levels indicative of tumor lysis syndrome were detected, requiring application of rasburicase and intravenous hydration. After stabilization of serum creatinine values, serological assessment of disease activity three weeks after CAR T-cell infusion already showed decrease of serum kappa free light chains from 3328 mg/l at admission to 4 mg/l and an unprecedented depth of remission (VGPR, see Figure 1). Currently, the patient's state of remission is still improving. Updated follow-up and data on CAR T-cell expansion and persistence will be presented at the meeting.
Our case illustrates rapid response to anti-BCMA CAR-T cells (Ide-cel) after failing treatment with an ADC and a bispecific antibody directed against BCMA. This demonstrates that sequential treatment can be effective even after multiple prior lines of anti-BCMA directed therapies. Patients with prior exposure to anti-BCMA agents should have access to anti-BCMA CAR T-cells.
Disclosures
Merz:Janssen: Honoraria; BMS Celgene: Honoraria. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Metzeler:Daiichi Sankyo: Honoraria; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy, Honoraria, Research Funding; Curis: Research Funding; Astellas: Honoraria; AbbVie: Honoraria. Schwind:Novartis: Honoraria. Herling:Abbvie: Honoraria, Research Funding; EDO-Mundipharma: Honoraria, Research Funding; Janpix: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Jazz: Honoraria, Research Funding. Jentzsch:Pfizer: Honoraria; Jazz Pharmaceuticals: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal